Introduction
Sepsis is a condition marked by life-threatening organ dysfunction resulting from a prolonged imbalance of the innate immune response to infection. Presently, it stands as the primary cause of mortality in intensive care units [1]. However, to date, there are no effective treatment strategies against sepsis. The excessive release of inflammatory cytokines during severe infections is a key factor in sepsis development [2]. Therefore, the use of anti-inflammatory agents against sepsis is a promising treatment option.
Urinary trypsin inhibitor (UTI), widely recognized as ulinastatin, is a bioactive compound sourced from human urine. Renowned for its unique ability to inhibit enzyme activity, UTI plays a pivotal role in effectively alleviating the systemic inflammatory response. This multifaceted inhibitor finds common therapeutic use in diverse medical scenarios, including the treatment of patients with pancreatitis, traumatic injuries, cardiopulmonary bypass procedures, and those undergoing cancer chemotherapy [3, 4]. UTI has protective properties against protamine-induced myocardial damage by inhibiting tumor necrosis factor α (TNF-α) synthesis [5]. Moreover, UTI demonstrates both anti-inflammatory and antioxidant effects in LPS-treated RAW264.7 cells. This is achieved by suppressing the c-Jun N-terminal kinase (JNK)/nuclear factor kappa B (NF-κB) signaling pathway and activating the PI3K/Akt/Nrf2 pathway [6]. A meta-analysis showed that UTI is significantly beneficial for patients with ARDS by reducing serum inflammatory factors, including TNF-α, interleukin (IL)-1β, IL-6, and IL-8 [7].
However, the actual protective mechanism of UTI against immune cell inflammation is still not completely understood. Bacterial lipoprotein (BLP) is a vital constituent of the cell wall in both gram-positive and gram-negative bacteria. Activation of monocytes and macrophages by BLP results in the production of inflammatory cytokines [8]. Toll-like receptors (TLRs), a family of immune cell receptors, play a crucial role in recognizing components of the bacterial cell wall through pattern recognition receptors [9]. Hence, the objective of this research was to examine the effect of UTI in septic mice and to elucidate the molecular mechanism responsible for its anti-inflammatory effect on BLP-induced THP-1 cells.
Material and methods
Animal and reagents
The experiment was conducted at Nanjing Medical University and lasted for one year, including animal research and cell experiments. The Laboratory Animal Ethics Committee and the Laboratory Animal Center of Nanjing Medical University approved the animal experiment protocol. Forty male C57BL/6J mice, with a weight of 22 ±0.3 g, were acquired from Nanjing Medical University (Nanjing, China). The mice were accommodated and nourished in a controlled environment with regulated temperature and humidity, following a standard light/dark cycle of 12 hours each for one week. During this period, they were provided with unrestricted access to both water and food. All procedures involving animals were executed as per the guidelines outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals. UTI was obtained from Guangdong Techpool Bio-Pharma Co., Ltd. (Guangdong, China). The THP-1 cell lines were acquired from the Shanghai Institute of Biological Products Co., Ltd. (Shanghai, China).
Cecal ligation and puncture
Cecal ligation and puncture (CLP) serves as a polymicrobial sepsis model, closely stimulating human polymicrobial sepsis. It has been widely adopted by researchers to explore the pathology of sepsis. The original CLP protocol described in previous studies was followed [10, 11]. In brief, mice were administered anesthesia by injecting sodium pentobarbital (50 mg/kg, Sigma-Aldrich, USA) intraperitoneally prior to surgical interventions. A midline laparotomy was carried out, involving a 5-cm incision along the midline. Following this, the cecum was exposed, and a ligature was applied just below the ileocecal valve. The cecum was carefully extracted. Subsequently, a ligature was applied to the distal 30% of the cecum. Afterward, two punctures were made in the cecum using a sterile 20-gauge needle, followed by gentle squeezing to extrude fecal material from the wounds. The cecum was then repositioned, followed by the meticulous layer-by-layer repair of the abdominal cavity. Mice received resuscitation through a subcutaneous solution (3 ml/100 g body weight).
Forty mice were assigned randomly to four groups: control (n = 10), sham operation (n = 10), CLP (n = 10), and CLP + UTI (5000 U/kg, n = 10). Sham group animals underwent a sham operation instead of CLP. Control group animals did not undergo surgical treatment. After surgery, mice assigned to the CLP + UTI group were treated with 5000 U/kg (intraperitoneal injection) UTI. Control, sham- operation and CLP groups were injected with the same volume of normal saline.
Histopathological examination of the heart and lung
The hearts and lungs of mice were harvested 24 h later and examined for morphological signs of injury after CLP to assess the efficacy of UTI in reducing multiple organ damage. Heart and lung tissue samples underwent fixation in 4% paraformaldehyde for 48 hours. Following dehydration, the samples were embedded in paraffin. Subsequently, 5-µm sections were prepared and underwent staining using hematoxylin and eosin (Sigma-Aldrich, USA). Stained images were captured at 200× magnification via optical microscopy (Nikon Eclipse E100, Nikon, Japan).
Transmission electron microscopy
Hearts were removed, and small tissue blocks from the middle section of the left ventricular wall were fixed in 2% osmium tetroxide (OsO4) in PBS (pH 7.4) with 1.5% potassium ferricyanide. After dehydration with an ethanol gradient, the tissues were embedded with propylene oxide as an intermediary solvent. Ultrathin sections (50 to 70 nm) were stained with uranyl acetate and lead citrate. The images were examined under a Hitachi H-7650 electron microscope (Hitachi, H-7650, Tokyo, Japan).
Serum biochemical analysis
Blood plasma was collected from mice 24 hours after CLP surgery. Fresh blood samples were centrifuged at 3000 rpm at 4oC for 15 minutes, after which the plasma supernatant was collected and stored at –80°C for subsequent analyses. The plasma concentrations of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were measured using an OLYMPUS AU640 automatic analyzer (Tokyo, Japan) according to the manufacturer’s instructions.
Cell lines and culture
Experiments were performed utilizing the human monocyte cell line and THP-1 cells (Shanghai, China) after BLP (Sigma-Aldrich, USA) stimulation. THP-1 cells were cultured in RPMI 1640 medium (Gibco, USA) comprising 10% heat-inactivated fetal bovine serum (Gibco, USA), penicillin (100 units/ml), streptomycin sulfate (100 µg/ml), and glutamine (2.0 M). The culture was maintained in a humid environment at 37oC in 5% CO2.
Cytokine analysis
THP-1 cells (2 × 105 cells/well) were cultured in 24-well plates. Cells in the control group were exposed to an incomplete RPMI 1640 medium. In contrast, the treated cells were subjected to 1,000 ng/ml of BLP for 4 hours. THP-1 cells were stimulated with UTI at varying concentrations (10, 100, and 1000 U/ml) alongside 1,000 ng/ml BLP, and the incubation procedure was carried out for the same duration. Following the incubation, the cell supernatant underwent collection and was promptly subjected to centrifugation at 3,000 rpm for 10 minutes at 4oC.
According to the instructions provided by the manufacturer for enzyme-linked immunosorbent assay (Proteintech, China), the microplate reader was employed to measure the absorbance of the samples. Subsequently, a standard curve was generated. The concentrations of cytokines IL-1β and TNF-α in both the serum and supernatants of THP-1 cells were then calculated utilizing the formula derived from the standard curve.
Western blot analysis
THP-1 cells (2 × 106 cells/well) were grown and divided into the control, BLP, and BLP + UTI (10, 100, and 1000 U/ml) groups. After two cold PBS washes, the cells underwent lysis using a buffer comprising 1.0% Nonidet P-40, 150 mM NaCl, 10 mM Tris-Cl (pH 7.5), and 1 mM EDTA. The resulting supernatant, representing the cytoplasmic fraction, was transferred into a microcentrifuge tube that had been precooled and kept at –80oC for further use. The nuclear pellet was reconstituted in lysis buffer containing dithiothreitol, lysis buffer, and a protease inhibitor cocktail with subsequent preparation of the supernatant (nuclear fraction) following the methodology outlined in prior research. The protein concentrations in the extracts were measured utilizing the bicinchoninic acid (BCA) protein assay. Equivalent quantities of protein then underwent sodium dodecyl-sulfate polyacrylamide gel electrophoresis with subsequent transfer to Immobilon-P membranes (Millipore, Bedford, MA). The membranes underwent blocking for 1 hour at room temperature using 5% fat-free milk. Subsequently, they were probed overnight (kept at 4oC) with the relevant primary antibodies, following the manufacturer’s instructions (anti-NF-κB p65, anti-IκB, phosphorylated specific anti-IκB, P-κB, P-P38, and P-38; Cell Signal Technology, the USA). Subsequently, the membranes were rinsed with Tris-buffered saline with Tween and exposed to the respective secondary antibodies (Abcam; dilution 1 : 10,000) for 1 hour at room temperature. The fluorescence signal was captured utilizing the Bio-Rad imaging system (Bio-Rad Laboratories, Hercules, CA, USA). Signal intensities were then quantified utilizing ImageJ (Wayne Rasband, USA).
Statistical analysis
Data were presented as mean ± standard deviation. The comparison of data across more than two groups was performed by means of a one-way analysis of variance, followed by Tukey’s test. Statistical analysis was performed utilizing GraphPad Prism (V9.0 Prism, La Jolla, CA). P < 0.05 was considered a statistically significant value.
Results
UTI administration relieves myocardial pathological damage, improves pathological damage to the lung tissue, and ameliorates myocardial mitochondrial damage
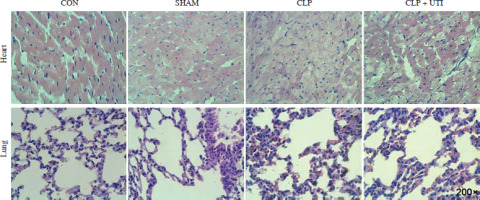
Hematoxylin and eosin staining (Fig. 1) showed that in comparison to the control and sham operation groups, the CLP group exhibited myocardial tissue injury and inflammation. This was evident from increased tissue edema and elevated neutrophil infiltration levels. Similarly, CLP induced significant neutrophil infiltration in the lung tissue. However, the pathological changes in the lung tissue caused by sepsis remarkably decreased in CLP mice treated with UTI.
Fig. 1
Hematoxylin & eosin (HE) staining was used to determine the pathological changes in the myocardial tissue and the lung tissue in the control, sham operation, CLP, and CLP + UTI groups. HE staining showed normal cardiomyocyte morphology and regular arrangement of myocardial fibers in the control group and apparent myocardial injury in the CLP-induced sepsis group (magnification 200×). CON – control group, SHAM – sham operation group, CLP – cecal ligation and puncture group, CLP + UTI – cecal ligation and puncture + urinary trypsin inhibitor UTI (5000 U/kg) administration group
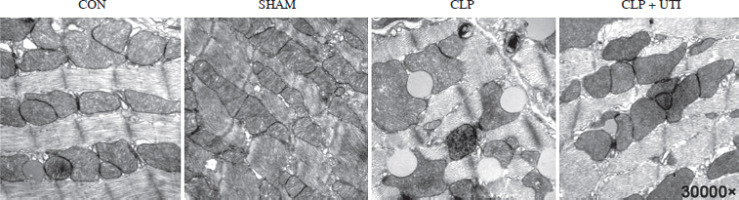
Transmission electron microscopy analysis was conducted to visualize the morphology of mitochondria. As shown in Figure 2, the cecal ligation and puncture operation induced mitochondrial swelling, ruptured cristae, and lipid droplet formation. However, administration of UTI resulted in improved mitochondrial injury compared to the CLP group.
UTI treatment reduced the levels of myocardial injury biomarkers and the serum IL-1β levels in septic mice
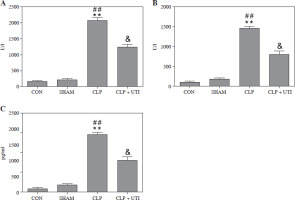
To examine the impact of UTI on myocardial injury and the inflammatory response in septic mice, the CLP model of polymicrobial sepsis was established, as described in a previous study [12]. The outcome (Fig. 3) revealed a considerable increase in the levels of CK-MB and AST, recognized as biomarkers of myocardial injury, along with an increase in the level of pro-inflammatory cytokine IL-1β in CLP mice. Nevertheless, the levels of these indicators were reduced in mice treated with UTI. The group treated with UTI exhibited improved heart injury and inflammatory marker enzyme activities. Hence, UTI has significant cardioprotective and anti-inflammatory activities.
Fig. 3
Changes of myocardial enzyme indexes and serum IL-1β in mice from each group. A) Muscle kinase isoenzyme (CK-MB); B) Aspartate aminotransferase (AST); C) Interleukin 1β. Plasma levels of CK-MB, AST, and IL-1β in the cecal ligation and puncture (CLP) group were markedly higher than those in the control group and the sham operation group. Urinary trypsin inhibitor (UTI) treatment significantly reduced CK-MB, AST, and IL-1β levels from the CLP + UTI group compared with the CLP group (n = 10). Values are expressed as mean ± standard error of the mean. **p < 0.01 vs. control group, ##p < 0.01 vs. SHAM group, &p < 0.05 vs. CLP group. CON – control group, SHAM – sham operation group, CLP – cecal ligation and puncture group, CLP + UTI – cecal ligation and puncture + urinary trypsin inhibitor (5000 U/kg) administration group
UTI improves the expression of TNF-α and IL-1β induced by BLP in THP-1 cells
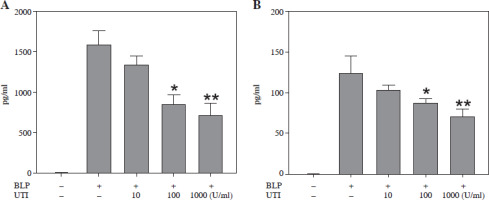
Bacterial lipoprotein is a key component of the bacterial wall and has been found to activate different types of cells, encompassing macrophages, neutrophils, lymphocytes, endothelial cells, and epithelial cells, via the TLR2 signaling pathway [9]. In the experiments of this study, BLP was utilized to stimulate THP-1 cells and establish a sepsis model. The development of inflammation is remarkably influenced by pro-inflammatory cytokines. The measurement of IL-1β and TNF-α was conducted to assess how UTI affected the levels of inflammation in THP-1 cells induced by BLP. In Figure 4, it can be observed that THP-1 cells subjected to BLP treatment demonstrated remarkably elevated expression levels of TNF-α and IL-1β in cell supernatants compared to the control group. Nevertheless, when THP-1 cells were exposed to UTI at various concentrations, there was a dose- dependent reduction in TNF-α and IL-1β levels. Based on these findings, UTI has anti-inflammatory effects in vitro.
Fig. 4
Urinary trypsin inhibitor (UTI) attenuates bacterial lipoprotein (BLP)-induced TNF-α (A) and IL-1β (B) expression in THP-1 cells. Cells were treated with UTI (10, 100, 1000 U/ml) and stimulated with BLP (100 ng/ml). After 4 h stimulation, cell supernatant was collected and measured via ELISA. Data were analyzed by one-way ANOVA followed by Tukey’s multiple comparisons test (n = 3). *p < 0.05, **p < 0.01 vs. BLP stimulated THP-1 cells
Comparison of the NF-κB, P-IκB, and P-P38 expression in THP-1 cells induced by BLP stimulation
To investigate intracellular signaling cascades, focus was placed on the NF-κB/I-κB and P38/mitogen-activated protein kinase (MAPK) pathways, which have an important role in inflammation [13, 14]. Based on the data presented in Figure 5A, the BLP-induced group had significantly higher expression of the nucleoprotein NF-κB in comparison to the control group. Nevertheless, a prominent decreasing trend was observed in the expression of THP-1 cells when subjected to UTI in a dose-dependent manner. Similarly, the expression of cytoplasmic protein P-IκB was significantly elevated after BLP stimulation in comparison to the controls. However, it exhibited a gradual decrease in a concentration-dependent manner following UTI treatment. In contrast, the IKB protein exhibited an opposite trend (Fig. 5B).
Fig. 5
Urinary trypsin inhibitor (UTI) ameliorates inflammatory responses by inhibiting the NF-κB and P38/MAPK pathways. A) Effects of different concentrations of UTI on expression of NF-κB in the nucleus of THP-1 cells stimulated by bacterial lipoprotein (BLP). Western blot method was used to detect the expression level of NF-κB in the nuclear protein of THP-1 cells (left column); quantification of western blotting results presented in right column (n = 3). P-values were determined by one-way ANOVA followed by Tukey’s test (**p < 0.01 vs. control group and #p < 0.05 vs. BLP group). B) The impact of various concentrations of UTI on expression of p-IκB and IκB in cytoplasmic proteins of THP-1 cells, stimulated by BLP, was investigated. Levels of p-IκB and IκB in cytoplasmic proteins were detected using the western blot method after inducing THP-1 cells with BLP. Quantification of western blotting results presented in right column (n = 3). P-values were determined by one-way ANOVA followed by Tukey’s test (**p < 0.001 vs. control group and ##p < 0.01 vs. BLP stimulated group). C) Phosphorylated levels of P38 were determined in BLP-induced THP-1 cells with or without UTI. Quantification of western blotting results presented in right column (n = 3). P-values were determined by one-way ANOVA followed by Tukey’s test (**p < 0.01 vs. control group and #p < 0.05 vs. BLP stimulated group)
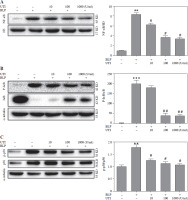
Subsequently, BLP stimulation of THP-1 cells caused an increase in P38 phosphorylation, and UTI reduced P38 phosphorylation in a concentration-dependent manner (Fig. 5C).
Discussion
Sepsis, an inflammatory disease mediated by activation of the innate immune system, is characterized by the excessive release of inflammatory cytokines [15]. TLRs play an important role in recognizing danger signals, both from external and internal sources. Activation of TLRs triggers signaling pathways comprising the MAPK, NF-κB, and interferon-responsive factors, leading to the secretion of pro-inflammatory mediators encompassing TNF-α and IL-1β [16, 17]. These cytokines are commonly used to assess the inflammation level in sepsis. The release of pro-inflammatory cytokines results in systemic inflammatory responses and dysfunction of various organs, particularly the heart and lungs, leading to multiple organ dysfunction syndrome. In this study, sepsis-induced myocardial injury was indicated by significantly higher levels of CK-MB and AST, along with pathological alterations observed in the myocardial tissue after CLP. Similarly, lung tissue pathology confirmed sepsis-induced lung tissue damage. Further, other studies have revealed that UTI has an organ-protective function, as observed in the current study [18, 19].
Interleukin 1β, a regulatory factor of cytokines and polypeptides, is produced by monocytes and macrophages, playing a crucial role in cellular immune activation. It has various functions in the hematopoietic system, nervous system, endocrine system, inflammatory response, and certain antitumor processes in the body. Abnormal synthesis of IL-1β in the local tissue can cause fever and inflammation. Moreover, IL-1β is a major inducer of the acute phase reaction and can be stimulated by various factors such as antigens, endotoxins, bacteria, and viruses [20, 21]. Earlier research has indicated that the release of IL-1β is linked to myocardial and lung injury [18, 19]. The current study revealed that UTI decreased IL-1β release, which is consistent with previous findings. In this study, in vitro experiments were executed to validate the role of UTI in reducing the maximum secretions of TNF-α and IL-1β triggered by BLP. These effects were observed in septic mice and were concentration-dependent. The results aligned with the previous research findings [19, 22]. Similarly, it was observed that UTI decreased the expression of inflammatory factors in THP-1 cells stimulated by BLP in a concentration-dependent manner. Furthermore, UTI not only reduces the levels of pro-inflammatory factors in septic mice but also decreases neutrophil infiltration in the heart and lung tissues, attributable to its anti-inflammatory effects.
Urinary trypsin inhibitor has been proposed as a potential myocardial protective agent. Previous studies have reported that ulinastatin can mitigate lipopolysaccharide-induced cardiac dysfunction by inhibiting NLRP3 inflammasome activation [19]. CK-MB and AST are essential serum cardiac biomarkers evaluating myocardial injury. Mitochondria play an important role in cardiomyocytes by providing energy to the cells and triggering apoptosis [23]. In our present study, septic mice were treated with UTI, and the results indicated a protective effect of UTI on the hearts of septic mice.
The presence of BLP is important in virulence in several gram-positive bacteria and is highly immune stimulatory by triggering inflammatory TLR2 signaling. After TLR2 activation, IkBα is phosphorylated, and NF-κB is released to promote the transcription of inflammatory genes [24- 26]. NF-κB is involved in several pathways that modulate inflammation. If stimulated by external factors, IkB is phosphorylated (mediated by Iκb kinase) and dissociated from the inactivated NK-κB-IkB complex, resulting in NF-κB activation. It enters the nucleus to bind to the corresponding DNA sequences of inflammatory cytokine genes, thereby regulating the expression of inflammatory cytokines [27, 28].
This investigation showed that UTI could effectively inhibit the BLP-induced inflammatory response in THP-1 cells via the classical pathway of nuclear transcription factor. In particular, UTI inhibited IkB phosphorylation in the cytoplasm, resulting in a reduction in activated NF-κB entering the cell nucleus. This mechanism can effectively suppress the expression of acute inflammatory cytokines, thereby controlling an excessive inflammatory response.
The MAPK signaling pathway, which involves various proteins such as P38, ERK1/2, and JNK1/2, plays an important role in inflammation. This pathway is responsible for secreting pro-inflammatory cytokines and the interaction between these proteins, each of which plays a different role in the inflammatory response. Previous research has shown that activation of the ERK1/2 and P38 pathways promotes the release of TNF-α during endotoxemia. Moreover, JNK can reduce TNF-α production and improve cardiac function by inhibiting ERK1/2 and P38 activation [29]. Past investigations have indicated that the MAPK signaling pathway participates in sepsis development and is closely associated with cytokine secretion [30, 31]. This research showed that P38 phosphorylation in the MAPK signaling pathway was significantly activated in THP-1 cells after BLP stimulation. Meanwhile, UTI treatment significantly inhibited P38 phosphorylation. Hence, UTI demonstrates therapeutic efficacy in alleviating inflammation by suppressing the production of pro-inflammatory cytokines via the P38/MAPK pathway.
Conclusions
Urinary trypsin inhibitor administration can reduce sepsis induced by CLP and THP-1 cells stimulated by BLP. This reduction is linked to attenuated production of pro-inflammatory factors. Hence, these effects can be attributed, at least in part, to downregulation of p-P38 expression and inhibition of the NF-κB pathway.