Introduction
Acne is an inflammatory skin disease with a high incidence in adolescents [1]. Cutibacterium acnes is one of the causes of acne inflammation. It affects the pilosebaceous units of skin, resulting in inflammatory skin diseases such as acne, whose symptoms primarily appear as pustules, papules, nodules, and other inflammatory lesions [2, 3]. Therefore, understanding the molecular mechanism of acne pathogenesis triggered by C. acnes is vital for creating effective treatment strategies.
Circular RNAs (circRNAs) are a class of RNA molecules formed through an atypical reverse splicing mechanism with a closed ring structure connected by covalent bonds. This circular structure enhances their stability compared to linear RNA molecules. Although some circRNAs have been found to code for proteins, they are classified as non-coding RNAs due to the lack of this function in previously discovered circRNAs [4]. Recent studies suggest that circRNAs may contribute to the initiation and progression of acne. Liang et al. identified 538 differentially expressed circRNAs (271 up-regulated and 267 down-regulated) in lesional and non-lesional skin of severe acne patients. Their RT-qPCR analysis confirmed significantly reduced expression of five circRNAs (circRNA_0084927, circRNA_0001073, circRNA_0005941, circRNA_0086376, and circRNA_0018168) in severely acne-damaged skin. These circRNAs were predicted to regulate the expression of downstream genes by binding numerous microRNAs (miRNAs) in acne [5]. MiRNAs are a group of non-coding RNA molecules, about 21 to 23 nucleotides long, that can block the post-transcriptional translation of target genes by binding to their mRNAs specifically to participate in the occurrence of inflammatory diseases [6, 7]. It has been shown that circRNAs can influence human inflammatory skin diseases by managing gene expression via the circRNA-miRNA-mRNA network. Defective regulatory T cells (Tregs) are associated with the onset and progression of psoriasis [8]. Circ_0003738 acts as a sponge for miR-490-5p, weakening its inhibition of the target gene IFNGR2, which undermines Treg function and leads to psoriasis development [9]. Conversely, circRAB3B can hinder psoriasis progression by sponging miR-1228-3p to upregulate PTEN [10]. In acne, hsa_circ_0105040 enhances the expression of IRAK1 and TRAF6 by sponging miR-146a, leading to increased levels of inflammatory factors [11]. Therefore, targeting the circRNA-miRNA-mRNA network could be a promising approach for developing new acne treatments.
Hsa_circ_0001445 regulates miRNA-640, inhibiting the inflammatory responses induced by oxidized low-density lipoprotein (ox-LDL) in human umbilical vein endothelial cells (HUVECs) [12], but it remains unknown in acne. MiR-1298-5p has been reported to be involved in the inflammatory response of fibroblasts in rheumatoid arthritis, where it promotes E2F1 expression through being sponged by OSER1-AS1, thus reducing the levels of inflammatory factors [13]. Genetic analysis revealed estrogen receptor alpha (ESR1) gene polymorphism in acne patients. ESR1 may be used as a drug target to regulate the metabolism and inflammatory response of skin cells and improve the cellular immune microenvironment, thereby treating acne [14, 15]. In this study, we aimed to investigate whether hsa_circ_0001445 can regulate the expression of ESR1 by sponging miR-1298-5p, thereby playing a role in acne.
Material and methods
Bioinformatics analysis
The GSE212605 dataset, obtained from the GEO database (https://www.ncbi.nlm.nih.gov/geo/), features differential expression data of circRNA, miRNA, and mRNA. These data were gathered through RNA microarray analysis of biopsy specimens from the pathological tissue of six patients with severe acne undergoing surgery, as well as the healthy facial skin of six volunteers undergoing facial plastic surgery. Downstream miRNAs of hsa_circ_0001445 were sourced from the starBase database (https://rnasysu.com/encori/index.php). The Circular RNA Interactome database (https://circinteractome.nia.nih.gov/index.html) was used to predict the binding site of hsa_circ_0001445 and miR-1298-5p. The target genes of miR-1298-5p were predicted the through starBase database. The mRNAs associated with acne were retrieved from the GeneCards database (https://www.genecards.org/).
Activation of C. acnes
Cutibacterium acnes from ATCC (ATCC11827) was incubated at 37°C for 72-96 hours to form colonies. A single colony of C. acnes was then transferred into a 1.5 ml EP tube containing 1 ml of brain heart infusion (BHI) and incubated under anaerobic conditions at 37°C for 48 hours. Subsequently, bacterial sediment was collected by centrifugation (3000 × g for 10 minutes) at 4°C. This sediment was re-suspended in a 50 ml centrifuge tube with 20 ml of BHI and cultured under the same conditions for 72 hours. After another round of centrifugation at 4°C, the bacterial sediment was diluted to 1 × 108 CFU/ml with BHI for the subsequent biofilm formation experiment [16].
Acquisition of biofilm-derived C. acnes (Bio-C. acnes)
A sterile plastic cell culture cap with a 13-mm diameter was placed into each well of a 24-well plate to serve as the growth surface for biofilm formation. 1 × 108 CFU/ml bacterial suspension (500 µl) of C. acnes was added to each well. The plate was then placed in anaerobic conditions at a temperature of 37°C. The BHI medium was replaced every other day. After 8 days, the biofilm was scraped from the cell culture plate and re-suspended in a PBS buffer. The suspension was agitated repeatedly with a pipette and a shaker to disperse the bacteria. The biofilm bacteria sample was then diluted to 2 × 108 CFU/ml in PBS and heat-treated at 80°C for 30 minutes [16]. This process resulted in inactivated Bio-C. acnes, which was then used for cell stimulation.
Cell culture and stimulation
Human keratinocytes and sebocytes were procured from the American Type Culture Collection (ATCC) being cultured with DMEM medium (Gibco, Grand Island, USA) supplemented with 10% FBS, 1% streptomycin, and 1% penicillin in a sterile, humid environment at 37°C with 5% CO2. Keratinocytes and sebocytes with a cell density of 80% were then treated with Bio-C. acnes for 24 hours to trigger an inflammatory response [16].
RT-qPCR
Total RNA was extracted using the TRIzol Plus RNA purification kit (Thermo Fisher, Shanghai, China) and reverse-transcribed into cDNA using the RevertAid RT reverse transcription kit (Thermo Fisher, Shanghai, China) for PCR, following the kit instructions. The levels of hsa_circ_0001445 and ESR1 mRNA were normalized by GAPDH, and the miR-1298-5p level was normalized by U6. Their fold changes were calculated using the formula 2ΔΔCt. The sequences of primers used for RT-qPCR are listed in Supplementary Table S1.
Transfection
Keratinocytes and sebocytes were prepared in a 6-well plate a day in advance. The cell density was set at 5 × 105 cells/well for plasmid transfection and 2 × 105 cells/well for RNA transfection. The Lipo6000 Transfection Reagent (Beyotime, Shanghai, China) and the hsa_circ_0001445 overexpression plasmid or miR-1298-5p mimics (mim-1298-5p) were diluted separately and left to stand at room temperature for 5 minutes. The two solutions were then combined and allowed to sit at room temperature for another 5 minutes. This transfection reagent mixture was added to the 6-well plate (250 µl/well). After 6 hours, the transfection reagent was replaced with fresh serum medium. The cells were cultured for an additional 24-48 hours before subsequent experiments.
ELISA
The cell culture supernatant was collected for ELISA analysis. The ELISA kit (Solarbio, Beijing, China) was used to measure the secretion levels of interleukin (IL)-6, IL-8, and tumor necrosis factor α (TNF-α).
Double luciferase reporting assay
Luciferase vectors (400 ng/ml) of hsa_circ_0001445 wild-type (wt-circ1445), hsa_circ_0001445 mutation (mut-circ1445), ESR1 wild-type (wt-ESR1), ESR1 mutation (mut-ESR1), and empty vectors were each transfected into human keratinocytes and sebocytes along with 100 nM miR-1298-5p mimics or negative control were cotransfected. After lysing the cells, the supernatant was collected for fluorescence intensity detection using the microplate reader. The luminescence ratio of firefly and Renilla luciferase was calculated using a microplate reader, following the protocol of the Renilla-Firefly Luciferase Dual Assay Kit (MedChem Express, USA).
Statistical analysis
All experiments in this research were performed four times. The Student’s t test method was used to evaluate the significance of two sets of data and one-way ANOVA was used to evaluate the significance of multiple sets of data in GraphPad software. All results are presented as the mean ± standard deviation (SD). p < 0.05 was considered statistically significant.
Results
Hsa_circ_0001445 showed low expression in acne
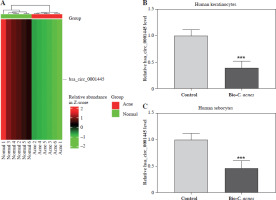
We derived a heat map of hsa_circ_0001445 expression from the GSE212605 dataset in the GEO database. The results showed that the level of hsa_circ_0001445 in the acne group was significantly lower than that in the normal group (p < 0.001, FC = 0.34) (Fig. 1A). To corroborate these findings, we examined hsa_circ_0001445 expression in cells. We found that its level was significantly down-regulated in Bio-C. acnes-stimulated human keratinocytes and sebocytes (Fig. 1B, C), suggesting that hsa_circ_0001445 may act as a negative regulator in the pathogenesis of acne.
Fig. 1
Hsa_circ_0001445 showed low expression in acne. A) The expression graph of hsa_circ_0001445 in acne patients and healthy volunteers from the GSE212605 dataset. B, C) The expression of hsa_circ_0001445 in normal or Bio-C. acnes-stimulated human keratinocytes and sebocytes (n = 4). ***p < 0.001. n – number of experiments
Hsa_circ_0001445 inhibits acne inflammation
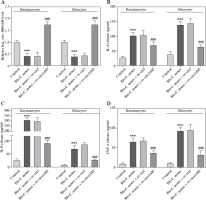
We sought to investigate the role of hsa_circ_0001445 in acne. To do this, we overexpressed hsa_circ_0001445 in Bio-C. acnes-stimulated human keratinocytes and sebocytes. The level of hsa_circ_0001445 was dramatically increased in the cell lines (Fig. 2A). We also found that the secretion levels of inflammatory cytokines (IL-6 and IL-8) and tumor necrosis factor (TNF-α) increased significantly in Bio- C. acnes-stimulated keratinocytes and sebocytes (Fig. 2B-D), which was reversed by the overexpression of hsa_circ_0001445 (Fig. 2B-D), illustrating that hsa_circ_0001445 has the potential to effectively inhibit acne inflammation.
Fig. 2
Hsa_circ_0001445 inhibits acne inflammation. A) Hsa_circ_0001445 level in Bio-C. acnes-stimulated human keratinocytes and sebocytes transfected with hsa_circ_0001445 overexpression vector (n = 4). B-D) Levels of IL-6, IL-8, and TNF-α in Bio-C. acnes-stimulated human keratinocytes and sebocytes transfected with or without hsa_circ_0001445 overexpression vector (n = 4). ***p < 0.001 (vs. control), ###p < 0.001 (vs. Bio-C. acnes + ov-ctrl). n – number of experiments
Hsa_circ_0001445 sponges miR-1298-5p
To better understand how hsa_circ_0001445 inhibits acne inflammation, we collected the miRNAs that are highly expressed in acne from the GSE212605 dataset. We then overlapped these with downstream miRNAs for hsa_circ_0001445. We found that three miRNAs (miR-5094, miR-127-5p, and miR-1298-5p) were shared (Fig. 3A), suggesting that these miRNAs may interact with hsa_circ_0001445. Notably, miR-1298-5p can induce the cellular inflammatory response [17]. A heat map of the expression data from GSE212605 showed that miR-1298-5p expression was higher in biopsy specimens from acne patients than that in healthy volunteers (Fig. 3B). Additionally, miR-1298-5p level was higher in Bio-C. acnes-stimulated keratinocytes and sebocytes compared to the control group (Fig. 3C, D). These results indicate that miR-1298-5p may promote acne pathogenesis. Then, we predicted the binding sequence of hsa_circ_0001445 and miR-1298-5p through the Circular RNA Interactome database (Fig. 3E).
Fig. 3
Hsa_circ_0001445 sponges miR-1298-5p. A) Venn diagram of high-expressed miRNAs in acne patients from GSE212605 dataset, as well as the target miRNAs of hsa_circ_0001445. B) Expression graph of miR-5094, miR-127-5p, and miR-1298-5p in acne patients and healthy volunteers from GSE212605 dataset. C, D) Expression of miR-1298-5p in normal or Bio-C. acnes-stimulated human keratinocytes and sebocytes (n = 4). E) Binding sequence of hsa_circ_0001445 and miR-1298-5p predicted using Circular RNA Interactome database. ***p < 0.001. n – number of experiments F, G) Effects of miR-1298-5p mimics on the luciferase activity of keratinocytes and sebocytes transfected with WT-circ1445 or MUT-circ1445 vectors (n = 4). ***p < 0.001. n – number of experiments


We constructed the wild-type (wt-circ1445) and mutant-type (mut-circ1445) luciferase vectors targeting the miR-1298-5p binding site. The luciferase activity was measured after co-transfecting with or without miR-1298-5p mimics in keratinocytes and sebocytes. We found that the luciferase activity of wt-circ1445 was suppressed by miR-1298-5p mimics, while that of mut-circ1445 was not significantly affected (Fig. 3F, G), indicating that hsa_circ_0001445 directly targeted miR-1298-5p. These findings demonstrate that hsa_circ_0001445 may alleviate acne inflammation through the regulation of miR-1298-5p by binding it.
Hsa_circ_0001445 relieved acne inflammation by targeting and negatively regulating miR-1298-5p
To further explore the regulatory effect of hsa_circ_0001445 on miR-1298-5p, we overexpressed hsa_circ_0001445 in Bio-C. acnes-stimulated keratinocytes and sebocytes. This resulted in a significant reduction in the level of miR-1298-5p (Fig. 4A), indicating that hsa_circ_0001445 negatively regulated miR-1298-5p expression in cells. Moreover, the overexpression of hsa_circ_0001445 also reduced the levels of IL-6, IL-8, and TNF-α in Bio-C. acnes-stimulated keratinocytes and sebocytes (Fig. 4B-D). However, these effects were reversed upon co-transfection with miR-1298-5p mimics. These results demonstrate that miR-1298-5p promoted the inflammatory response of acne, and hsa_circ_0001445 can alleviate this inflammation by targeting and negatively regulating miR-1298-5p.
Fig. 4
Hsa_circ_0001445 alleviates acne inflammation by targeting and negatively regulating miR-1298-5p. A) Effects of hsa_circ_0001445 overexpression or in combination with miR-1298-5p mimics on the hsa_circ_0001445 level of Bio-C. acnes-stimulated keratinocytes and sebocytes (n = 4). B-D) Effects of hsa_circ_0001445 overexpression or in combination with miR-1298-5p mimics on the levels of IL-6, IL-8, and TNF-α in Bio-C. acnes-stimulated keratinocytes and sebocytes (n = 4). **p < 0.01, ***p < 0.001 (vs. Bio-C. acnes + ov-ctrl), ##p < 0.01, ###p < 0.001 (vs. Bio-C. acnes + ov-circ1445 + mimNC). n – number of experiments
MiR-1298-5p directly bound and negatively adjusted ESR1 in acne
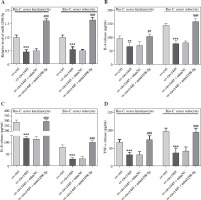
From the GSE212605 dataset, ESR1 was identified as a differentially expressed gene of acne which was shared with the genes targeted by miR-1298-5p (from the starBase database) and the genes associated with acne (from the GeneCards database) (Supplementary Fig. 1), suggesting that ESR1, closely related to acne, may be targeted and regulated by miR-1298-5p. We constructed wild-type (wt-ESR1) and mutant-type (mut-ESR1) luciferase vectors targeting the miR-1298-5p binding site (Fig. 5A). The luciferase detection results revealed that miR-1298-5p mimics significantly inhibited the luciferase activity of wt-ESR1, but had no effect on mut-ESR1 (Fig. 5B, C), illustrating that ESR1 was a target of miR-1298-5p. In addition, the mRNA level of ESR1 significantly decreased following the transfection of miR-1298-5p mimics in Bio-C. acnes-stimulated keratinocytes and sebocytes (Fig. 5D), indicating that miR-1298-5p negatively regulated the ESR1 expression in acne.
The levels of IL-6, IL-8, and TNF-α increased significantly in Bio-C. acnes-stimulated keratinocytes and sebocytes after transfection of miR-1298-5p mimics. However, this effect was reversed by the transfection of ESR1 plasmid (pcDNA-ESR1) (Fig. 5E, F). These results suggest that ESR1 can inhibit the inflammation induced by miR-1298-5p mimics and can be directly bound and negatively adjusted by miR-1298-5p in acne.
Fig. 5
MiR-1298-5p directly targets and negatively regulates ESR1. A) Binding sequence of miR-1298-5p and ESR1 mRNA predicted using starBase database. B, C) Effects of miR-1298-5p mimics on luciferase activity of keratinocytes and sebocytes transfected with WT-ESR1 or MUT-ESR1 vectors (n = 4). D) Effects of miR-1298-5p mimics alone or in combination with pcDNA-ESR1 on the mRNA level of ESR1 in Bio-C. acnes-stimulated keratinocytes and sebocytes after hsa_circ_0001445 overexpression (n = 4). E, F) Effects of miR-1298-5p mimics alone or in combination with pcDNA-ESR1 on IL-6, IL-8, and TNF-α levels in Bio-C. acnes-stimulated keratinocytes and sebocytes after hsa_circ_0001445 overexpression (n = 4). **p < 0.01, ***p < 0.001 (vs. Bio-C. acnes + ov-circ1445 + mimNC). ###p < 0.001 (vs. Bio-C. acnes + ov-circ1445 + mim1298-5p + pcDNA). n – number of experiments

Discussion
Acne is more prevalent in boys and girls in adolescence, which is harmful to the image of the patients, and causes a lot of psychological distress and pressure on the patients [18]. Currently, there are a variety of clinical methods to treat acne, including local treatment (topical drugs), systemic treatment (oral medicine) and physical therapy (laser therapy, etc.) [1]. However, they all have considerable limitations. Topical drugs can act directly on the affected area, but they are irritating to the skin and often cause discomfort to the patients. Oral drugs are mostly antibiotics and hormone drugs, long-term use of which may lead to drug tolerance or obesity in patients. Physical therapies such as laser therapy only serve as an adjunctive treatment for acne. Understanding the molecular regulation mechanism of acne pathogenesis and developing targeted drugs for typical molecules will improve the treatment effect of acne and reduce the side effects.
Cutibacterium acnes is a symbiotic skin bacterium, which is a major cause of acne inflammation. The presence of C. acnes biofilm-like structures in acne lesion tissue was verified by Jahns et al. [19-21]. These biofilm structures are more prevalent in the hair follicles of acne patients than in healthy volunteers, which partly explains the failure of antibiotic treatment in acne patients [22]. It has been shown that Bio-C. acnes activated TLR2 and its downstream NF-κB, p38, and ERK1/2 pathways in keratinocytes, and simultaneously induced production of the inflammatory mediators IL-6, IL-8, and TNF-α [16]. In this study, we constructed human keratinocyte and sebocyte inflammatory cell models using Bio-C. acnes to explore the pathogenesis of acne.
The circRNA-miRNA-mRNA network plays a significant role in inflammatory diseases. Identifying key circRNAs associated with the pathogenesis of acne and understanding the regulatory mechanisms of their downstream miRNA-mRNA are meaningful for acne treatment. In our research, we first identified hsa_circ_0001445 from the GSE212605 dataset as a low-expressed gene in acne patients. Hsa_circ_0001445 has been reported to be lowly expressed in a variety of human diseases including cancer and osteoporosis [23, 24], but its role in acne is unexplored. Cai et al. reported that hsa_circ_0001445 could inhibit ox-LDL-induced inflammatory responses in HUVECs by regulating miRNA-640 [12], suggesting a potential role of hsa_circ_0001445 in the development and progression of inflammatory diseases. We found that hsa_circ_0001445 levels were significantly down-regulated in Bio-C. acnes-stimulated keratinocytes and sebocytes. Its overexpression reversed the Bio-C. acnes-induced elevation of secretion levels of the inflammatory factors IL-6, IL-8, and TNF-α, suggesting that hsa_circ_0001445 has a potential to alleviate inflammatory responses in acne. MiR-1298-5p, predicted to be a downstream target of hsa_circ_0001445, was one of the highly expressed miRNAs in acne patients. MiR-1298-5p has been widely reported as a tumor suppressor [25, 26], but its role in acne is undefined. It was observed that miR-1298-5p increased the production of IL-6 and TNF-α in human bronchial epithelial cells by down-regulating SOCS6, leading to septicemic lung injury [17]. In rheumatoid arthritis, miR-1298-5p can be sponged by OSER1-AS1 to promote E2F1 expression, reducing the IL-1 and IL-6 levels of fibroblasts [13]. Our dual luciferase reporter gene assay confirmed the interaction between hsa_circ_0001445 and miR-1298-5p, suggesting that hsa_circ_0001445 may be able to alleviate the inflammatory responses of Bio-C. acnes-stimulated keratinocytes and sebocytes by targeting miR-1298-5p to regulate the expression of downstream genes. ESR1 is a predicted downstream gene of miR-1298-5p whose dysfunction triggers neuroinflammation [27]. The active ingredient in Jinhuang ointment regulates the inflammatory response of skin cells by acting on ESR1 [15], suggesting that ESR1 is a potential target for acne treatment. Our experiments showed that miR-1298-5p inhibited the expression of ESR1 by directly acting on its mRNA, while elevating the secretion levels of IL-6, IL-8, and TNF-α. Indeed, miR-5094 and miR-127-5p were also screened as downstream miRNAs of hsa_circ_0001445. HYOU1 and IGF1R were predicted to be the target genes of miR-1298-5p (Fig. 3A, Supplementary Fig. 1). Their mechanisms of action in the pathogenesis of acne will also be very valuable to study, and will be a part of our work in the future. Furthermore, in addition to keratinocytes and sebocytes, neutrophils and macrophages are also crucial participants in the pathogenesis of acne [28, 29]. Therefore, our future research will also focus on the roles of these immune cells in acne pathogenesis. More importantly, in the future, we will incorporate the necessary studies in vivo to further validate our findings.
In conclusion, this study identified a role for hsa_circ_0001445 in inhibiting the inflammation of Bio- C. acnes-stimulated human keratinocytes and sebocytes, which was achieved by sponging miR-1298-5p targeting ESR1. We will continue to investigate other key molecules in acne pathogenesis, providing a stronger theoretical basis for the development of new acne therapeutic strategies.


