Introduction
Breast cancer represents one of the most significant threats to women’s health worldwide [1]. The tumor microenvironment has been increasingly recognized as a crucial determinant of cancer invasion, progression, and clinical outcomes [2, 3]. Notably, breast cancers in Chinese populations present distinct clinical characteristics compared to Western populations, including larger tumor size and higher rates of human epidermal growth factor receptor 2 (HER2) overexpression [4], suggesting potentially more aggressive disease biology. While immunotherapy has revolutionized cancer treatment, response rates in breast cancer remain relatively modest, with objective response rates of only 5.3-6% even in triple-negative breast cancer [5, 6]. Particularly, while PD-1/PD-L1 inhibitors have shown promise in breast cancer treatment, response rates vary across molecular subtypes, with better outcomes observed in PD-L1-positive tumors and those with high TIL levels [7, 8]. Therefore, understanding the dynamic changes in PD-L1 expression and TIL profiles between primary tumors and metastatic sites is critical for optimizing immunotherapy strategies in breast cancer patients with recurrent disease.
A key mediator of tumor immune evasion is the programmed cell death-1 (PD-1)/programmed cell death ligand 1 (PD-L1) pathway. PD-1, expressed on T cells, interacts with PD-L1, which can be found on both tumor cells and immune cells. This interaction suppresses T cell activation and anti-tumor immune responses, thereby facilitating tumor progression [9-11]. The distribution of immune checkpoint molecules is complex, with PD-L1 and its related molecule PD-L2 being expressed on multiple immune cell types, including CD4+ and CD8+ T cells, monocytes, natural killer T cells, and B cells [10]. Notably, Ghebeh et al. discovered PD-L1 expression on tumor-infiltrating lymphocytes (TILs) in breast cancer [12]. Subsequently, studies revealed that PD-L1+ TILs can suppress T cell proliferation through reverse signaling [13] and modulate cytotoxic T lymphocyte (CTL) activity through PD-1 engagement [14]. The density and composition of TILs have significant prognostic implications across various cancers, with higher CD8+ T cell infiltration generally correlating with improved patient survival [15-18]. CD4+ T cells may also contribute to anti-tumor immunity through their helper and memory functions [17, 18]. However, critical knowledge gaps remain regarding how TIL populations and PD-L1 expression patterns change during metastatic progression, and how these changes influence patient outcomes. Understanding these dynamics in the context of post-surgical recurrence is particularly important for improving therapeutic strategies.
The dynamic interplay between tumor cells and immune cells creates distinct microenvironmental signatures in primary and metastatic lesions. Kim et al. documented significant differences in these immune landscapes between primary and metastatic sites [2], with metastatic lesions generally showing reduced TIL counts and decreased PD-L1 expression compared to primary tumors [3, 19-23]. A notable study by Ogiya et al. [3] examining 25 cases demonstrated that metastatic lesions in HER2-positive and triple-negative breast cancers exhibited lower percentages of TILs, CD8+ T cells, and CD4+ T cells compared to matched primary tumors, highlighting the role of immune escape in metastatic progression. Despite these insights, systematic studies comparing immune microenvironments between primary and postoperative recurrent breast cancer remain limited, particularly in Asian populations.
The current study investigated immune microenvironment differences between primary breast cancers and their matched metastatic lesions in Chinese women, with particular focus on distinct molecular subtypes. Our comprehensive analysis examined changes in immune parameters across disease sites, evaluated associations between TIL and PD-L1 expression patterns and clinical outcomes, and explored relationships between immune markers and molecular subtypes. Our aim was to address critical knowledge gaps in understanding breast cancer immunobiology in Asian populations and provide an evidence-based foundation for optimizing immunotherapeutic strategies in breast cancer treatment.
Material and methods
Patients and clinical samples
This study was conducted with approval from the Institutional Ethics Committee of the Second Affiliated Hospital of Nanchang University (Jiangxi, China) and adhered to the principles of the Declaration of Helsinki. We retrospectively identified 54 breast cancer patients diagnosed between May 2011 and December 2018 at our institution who subsequently developed localized or distant recurrence confirmed by pathological examination. Patient selection followed predefined inclusion and exclusion criteria. The inclusion criteria comprised: i) histopathologically confirmed breast cancer without other primary malignancies; ii) no history of anti-tumor therapy prior to initial diagnosis; iii) no anti-tumor therapy administered at time of recurrence diagnosis (only surgical removal of tumors was performed for both primary and metastatic lesions); iv) complete clinical follow-up documentation; v) availability of matched primary and recurrent tumor specimens. The exclusion criteria were: i) incomplete clinical data; ii) bilateral breast cancer; iii) male breast cancer; iv) advanced or metastatic disease at initial presentation or patients who underwent palliative surgery for locally advanced disease.
Immunohistochemical (IHC) analysis
For IHC analysis, tumor specimens were fixed in 10% formalin, embedded in paraffin, and sectioned at 4-µm thickness for hematoxylin and eosin (H&E) staining and IHC analysis. IHC was performed using primary antibodies against: estrogen receptor (ER) (1 : 150, clone OTI1B1, ZSBIO, China), progesterone receptor (PR) (1 : 150, clone I5E5, ZSBIO), HER2 (HercepTest, ZSBIO), CD3 (1 : 300; clone OTI3E10, ZSBIO), CD4 (1 : 50; clone B486A1, ZSBIO), CD8 (1 : 150, clone OTI3H6; ZSBIO), Foxp3 (1 : 100; clone 236A/E7; Talent Biomedical, China), and PD-L1 (1 : 500; Clone OTI9E12, ZSBIO). Hormone receptor positivity was defined as ≥ 1% of cancer cells expressing ER or PR. HER2-positive status by IHC was confirmed using fluorescence in situ hybridization (FISH). CD3, CD4, CD8, and PD-L1 expression was evaluated by membranous lymphocyte staining, while Foxp3 was assessed by nuclear staining. Staining intensity was scored as: none (0), mild (1; < 5% of tumor area), moderate (2; 5-50% of tumor area), or diffuse/severe (3; > 50% of tumor area) [20, 21]. ER/PR and HER2 status were classified according to ASCO guidelines [24, 25]. Peritumoral lymphoid aggregates were scored as: absent (0), focal (1+; rare isolated aggregates), present (2+; multiple aggregates), or well developed (3+; with germinal centers). Qualitative TIL scores were validated against digital quantification. Tumor cell PD-L1 membrane expression was scored in 1% increments (0-100%), with < 1% considered negative. Similarly, PD-L1+ TILs were scored as: none (0), focal (1+; < 5%), moderate (2+; 5-50%), or severe (3+; 51-100%) [26]. The stromal tumor-infiltrating lymphocytes (sTILs) scoring was performed according to the International TILs Working Group recommendations, evaluating the percentage of stromal tissue occupied by lymphocytes in hematoxylin and eosin-stained sections [27]. Tertiary lymphoid structures were evaluated using a standardized scoring system based on H&E-stained sections, where the presence and organization of lymphoid aggregates were assessed as: absent (0), focal (1+), present with germinal center formation (2+), or well developed with distinct T and B cell zones (3+) [28].
Statistical analysis
Statistical analyses were performed using SPSS version 20 (IBM, Armonk, NY, USA). Correlations between immunological features in primary tumors and matched metastatic lesions were assessed using the paired chi-square (χ2) test. Progression-free survival (PFS) and overall survival (OS) were evaluated using Kaplan-Meier analysis, with differences between survival curves compared using the log-rank test [26]. Statistical significance was set at p < 0.05.
Results
Baseline clinicopathological features of breast cancer patients
The clinicopathological features of enrolled patients (n = 54) are shown in Table 1. Patient age ranged from 24 to 76 years (median: 50 years), with 53.7% of patients younger than 50 years. The molecular subtypes comprised 16 cases (29.6%) of luminal type (ER/PR-positive, HER2-negative), 14 cases (25.9%) of HER2-positive, and 24 cases (44.5%) of triple-negative breast cancer. The predominant histological types were infiltrating ductal carcinoma (IDC) and invasive micropapillary carcinoma (IMC). Tumors were primarily high-grade, with 19 cases (35.2%) classified as grade 2 and 35 cases (64.8%) as grade 3. Notably, grade 3 tumors were more frequent in triple-negative (80%) and HER2-positive (64.3%) subtypes compared to luminal type (37.5%). Tumor size ranged from 0.7 to 9.4 cm (median: 2.5 cm), with 37.5% of cases presenting as multifocal disease. Disease stage distribution showed 6 cases (11.1%) at stage I, 23 cases (42.6%) at stage II, and 25 cases (46.3%) at stage III.
Table 1
Clinicopathological characteristics of primary surgical breast cancer specimens
Immunological profile of primary breast cancer specimens
The immune parameters are shown in Table 2. After excluding 4 cases with unsatisfactory primary tumor specimens, 50 cases were evaluated by immunohistochemistry. Of these, 13 cases (26%) were PD-L1-positive and 37 cases (74%) were PD-L1-negative in primary invasive tumors, while PD-L1 positivity was observed in 5 cases (33.3%) of ductal carcinoma in situ (DCIS). All primary tumors demonstrated stromal tumor-infiltrating lymphocytes (sTILs), with 9 cases (18%) showing mild infiltration (1+), 24 cases (48%) showing focal infiltration (2+), and 17 cases (34%) showing diffuse infiltration (3+). sTIL scores were notably higher in basal-like (95.5%) and HER2- positive (85.6%) subtypes compared to luminal type (60%). While triple-negative and HER2-positive breast cancers showed higher TIL levels than luminal type, they demonstrated lower CD8/FoxP3 to CD4/FoxP3 ratios (Fig. 1).
Table 2
Immune parameters of primary surgical breast cancer specimens
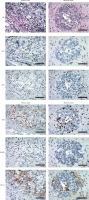
Fig. 1
Immunohistochemical comparison of immune markers in matched primary and metastatic breast cancer specimens. Representative images showing CD3, CD4 expression in paired primary breast tumor (left panels) and liver metastasis (right panels) from a single patient with basal-like subtype. Original magnification 400× Immunohistochemical comparison of immune markers in matched primary and metastatic breast cancer specimens. Representative images showing CD8, Foxp3, and PD-L1 expression in paired primary breast tumor (left panels) and liver metastasis (right panels) from a single patient with basal-like subtype. Original magnification 400×
Correlation of PD-L1+ TIL expression with clinical and immunological parameters in primary breast cancer
Table 3 presents correlations between PD-L1+ TIL intensity and clinical and immunological parameters in primary breast cancers. PD-L1+ TIL expression was classified as negative/low (0, 1) in 25 cases (50%), moderate (2) in 19 cases (38%), and diffuse (3) in 6 cases (12%). PD-L1+ TIL intensity showed significant positive correlations with tumor grade (R = 0.288, p = 0.043), tumor cell PD-L1 expression (R = 0.410, p = 0.004), and tertiary lymphoid structure score (R = 0.546, p < 0.001).
Table 3
The relationship of TIL PD-L1 expression with clinical and immune parameters in primary breast cancers
Relationships between PD-L1 expression, clinical parameters, and immune markers
Of the primary breast tumors analyzed, 37 cases (74%) were PD-L1-negative and 13 cases (26%) were PD-L1-positive. Associations between tumor cell PD-L1 expression and clinical/immune parameters were assessed using chi-square tests for categorical variables and the independent t-test for continuous variables (Table 4). While differences in PD-L1 expression were observed across molecular subtypes, correlations with clinical stage (p = 0.096), multifocal disease (p = 0.256), age (p = 0.212), tumor size (p = 0.232), and CD8/FoxP3 ratio (p = 0.13) did not reach statistical significance. However, significant associations were found between PD-L1+ TILs and molecular subtype (χ2 = 16.087, p = 0.003), tumor cell PD-L1 expression (χ2 = 8.887, p = 0.001), tertiary lymphoid structure score (χ2 = 38.372, p < 0.001), CD3+ T cell count (F = 10.920, p = 0.020), and CD4+ T cell count (F = 9.860, p = 0.035). Bivariate correlation analysis revealed that high PD-L1+ TIL expression positively correlated with adverse prognostic factors, including high tumor grade (r = 0.288, p = 0.043), tumor cell PD-L1 expression (r = 0.410, p = 0.004), and tertiary lymphoid structure score (r = 0.546, p < 0.001). These findings demonstrate significant associations between tumor cell PD-L1 expression, tumor grade, TIL density, and tertiary lymphoid structure development.
Table 4
The relationship of tumor cell PD-L1 expression with clinical and immune parameters in primary breast cancers
Survival analysis stratified by PD-L1 expression status
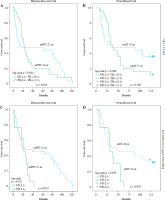
Kaplan-Meier survival analysis with log-rank test (Fig. 2) demonstrated that patients with low PD-L1+ TIL expression (scores 0-1) had median overall survival (OS) of 35 months compared to 37 months for those with high expression (scores 2-3). Median disease-free survival (DFS) was 16 months and 22 months for low and high PD-L1+ TIL expression groups, respectively. When comparing tumor cell PD-L1 status, PD-L1-negative patients showed longer median OS (48 months vs. 32 months) and slightly longer median DFS (22 months vs. 21 months) compared to PD-L1-positive patients. Overall survival rates were consistently higher in the PD-L1-negative group.
Fig. 2
Impact of PD-L1+ TILs and tumor cell PD-L1 expression on survival outcomes in primary breast cancer. A, B) Kaplan-Meier curves showing no significant association between PD-L1+ TILs and progression-free survival (PFS) or overall survival (OS). C, D) Kaplan-Meier curves demonstrating significantly shorter median PFS and OS in patients with PD-L1+ tumor cells compared to those with PD-L1– tumor cells
Analysis of metastatic lesions and PD-L1 expression patterns
Among the 50 cases with matched primary and recurrent breast cancer specimens, metastatic sites included chest wall/skin (24 cases, 48%), lymph nodes (12 cases, 24%), brain (4 cases, 8%), and lung (4 cases, 8%). As detailed in Table 5, PD-L1 protein expression was positive in 11 cases (22%), predominantly in chest wall/skin (7 cases), lung (2 cases), and lymph node metastases (2 cases), while brain and liver metastases showed minimal expression. The remaining 39 cases (78%) were PD-L1-negative. TILs were present in all metastatic lesions (100%), with scoring distributions of: mild (1+) in 31 cases (62%), moderate (2+) in 17 cases (34%), and severe (3+) in 2 cases (4%). PD-L1+ TIL expression was scored as negative/mild (0-1) in 43 cases (86%) and moderate (2+) in 7 cases (14%), with no cases showing severe (3+) expression. At median follow-up of 51 months (range: 1-133 months), 38 patients (76%) had died from metastatic disease.
Table 5
Characteristics of immunological parameters of matched metastatic sites
Comparison of immunological features between primary and metastatic lesions
Comparative analysis of immunological features between primary (n = 50) and recurrent (n = 50) breast cancer specimens is presented in Table 6. Chi-square tests were used to evaluate differences in immune parameters between primary and metastatic sites. Significant differences were observed in PD-L1+ TIL expression patterns between primary tumors and metastatic lesions. Additionally, tumor cell PD-L1 expression differed significantly between primary and metastatic sites, with TIL density scores showing marked variation (χ2 = 40.138, p < 0.001).
Table 6
Comparison of immunological features between primary and their matched metastatic tumors
Discussion
This study examined 54 patients with recurrent breast cancer who had not received neoadjuvant therapy. For context, Li et al.’s SWOG S0800 study [29] demonstrated that while neoadjuvant chemotherapy can alter the tumor immune microenvironment, TIL counts and PD-L1 expression remained stable in residual disease. Our cohort consisted of invasive ductal or micropapillary carcinomas, with median tumor size of 2.5 cm (53.7% of tumors between 2 and 5 cm). Disease staging showed 11.1%, 42.6%, and 46.3% of cases at stages I, II, and III respectively, with no stage IV cases due to the post-surgical nature of the cohort. Molecular subtype distribution in our study (29.6% luminal, 25.9% HER2-positive, 44.5% triple-negative) differed from Zheng et al.’s multicenter analysis of 4,211 Chinese breast cancer cases (1999-2008) [4], which reported 48.3-65.3% luminal, 18-25.5% HER2-positive, and 9.2-33.7% triple-negative cases. This disparity likely reflects our focus on recurrent disease, given the higher recurrence rates in HER2-positive and triple-negative subtypes compared to luminal cancers. These sampling differences should be considered when interpreting tumor microenvironment comparisons between primary and recurrent lesions.
Prior studies have shown higher TIL levels in triple- negative and HER2-positive breast cancers compared to HER2-negative tumors [30, 31]. Our findings align with this pattern: while TILs were present in all tumors, the proportion of cases showing focal (2+) or diffuse (3+) infiltration was notably higher in triple-negative (95.5%) and HER2-positive (85.6%) subtypes compared to hormone receptor-positive/HER2-negative tumors (60%). Analysis of TIL subsets revealed higher counts of CD3+, CD4+, CD8+, CD20+, and FoxP3+ lymphocytes in triple-negative and HER2-positive breast cancers compared to HER2-negative cases. These findings confirm previous observations that enhanced lymphocytic infiltration is characteristic of triple-negative and HER2-positive breast cancer subtypes. Recent single-cell and spatial transcriptomic analyses have revealed heterogeneous immune cell populations and distinct molecular signatures in metastatic sites [32]. Furthermore, while intratumoral TILs are generally associated with increased cell proliferation and improved survival, their relationship with chemotherapy response varies across different breast cancer subtypes [33]. These findings suggest that both the quantity and functional states of TILs, rather than mere presence, may be crucial for understanding their prognostic and therapeutic implications.
The PD-1/PD-L1 pathway has emerged as a crucial immune checkpoint in the tumor microenvironment [10]. PD-L1 protein expression occurs in both tumor-infiltrating immune cells and tumor cells [34, 35]. Reported PD-L1 expression rates vary widely across studies due to differences in sample sizes, sampling methods (tissue microarray versus whole sections), and detection techniques [3, 12, 20, 34, 35]. In our cohort, PD-L1 positivity was observed in 26% of tumor cells and 76% of TILs, falling within previously reported ranges of 21.7-51.6% for tumor cell expression and 41-81% for TIL expression [3, 12, 20]. Recent meta-analyses have demonstrated significant discordance in PD-L1 expression between primary and metastatic breast cancer [36], with particular relevance in triple-negative breast cancers [37]. Furthermore, comprehensive reviews have highlighted the prognostic implications of combined TILs and PD-L1 assessment from a pathological perspective [38]. These findings indicate that the PD-1/PD-L1 interaction between tumor cells and TILs represents a dynamic and complex relationship that evolves during disease progression, potentially influencing treatment response and patient outcomes.
Furthermore, our analysis of PD-L1+ TIL intensity and immunological characteristics in primary breast cancer revealed some unexpected findings. While previous studies [39-41] demonstrated associations between PD-L1+ TILs and molecular subtypes, with triple-negative breast cancers showing higher PD-L1+ TIL expression compared to hormone receptor-positive and HER2-positive cases, our correlation analysis did not confirm these relationships. This discrepancy may be attributed to methodological differences, including our use of whole tumor sections rather than tissue microarrays, and potential variations in PD-L1 antibody staining patterns. These technical factors might have contributed to higher PD-L1+ TIL ratios and intensities in luminal and HER2-positive cases than previously reported. However, our finding of a positive correlation between high PD-L1+ TIL expression and higher tumor grade aligns with previous studies [39-41].
The cellular distribution of PD-L1+ TILs remains controversial. While most studies report predominant PD-L1 expression in CD8+ T cells [10, 12, 20], Ghebeh et al.’s analysis of 44 breast cancer specimens using dual immunostaining demonstrated PD-L1 expression primarily in CD4+ T lymphocytes and its negative expression in Foxp3+ cells [39]. Our whole-slide digital image analysis revealed higher counts of CD3+ and CD4+ T cells in tumors with PD-L1+ TIL overexpression. This aligns with Schreiner et al.’s finding [42] that PD-L1-mediated immunosuppression partially involves CD4+ Treg lymphocytes. While breast cancer patients show increased immunosuppressive Treg cells in both peripheral blood and the tumor microenvironment, PD-1+ TILs predominantly express on CD3+ and CD8+ T cells [39, 43, 44]. These findings suggest that tumors with sufficient T cell infiltration, particularly CD3+ and CD4+ cells, may induce adaptive PD-L1 expression, potentially identifying patients who could benefit from PD-1/PD-L1 pathway blockade. Despite our limited sample size, these observations may help guide patient selection for PD-L1 inhibitor therapy.
Previous studies examining PD-L1+ TILs in matched primary and recurrent breast cancers are limited. Szekely et al. [21] reported reduced immune cell infiltration in metastatic lesions compared to primary tumors. Similarly, Cimino-Mathews et al. [20] observed decreased TIL density and PD-L1 expression in metastatic sites, particularly in triple-negative breast cancer. Our findings align with these observations and extend them by providing detailed characterization of PD-L1+ TIL patterns across different molecular subtypes in a Chinese cohort. Another meta-analysis further supports the prognostic significance of PD-L1+ TILs in primary versus metastatic breast cancer [45], highlighting their potential role in patient stratification for immunotherapy.
Our study has several limitations. First, the relatively small sample size and retrospective nature may limit the generalizability of our findings. Specifically, our limited sample size prevented comprehensive analysis of PD-L1+ TIL and PD-L1 expression patterns across different metastatic sites (chest wall, lymph nodes, lung, liver, and bone) and their relationships with clinicopathological factors (tumor grade, molecular subtypes, TIL density scores, and tertiary lymphoid structure development). Second, although we controlled for treatment-related confounding by including only surgically treated cases without prior or concurrent systemic therapy, these strict selection criteria might have introduced selection bias and may not represent the broader breast cancer population who receive various forms of treatment. Third, we only analyzed PD-L1 expression and TILs at a single timepoint in metastatic lesions, which may not fully capture the dynamic changes in the immune microenvironment [46]. Future studies should consider longitudinal sampling and more comprehensive immune profiling. Additionally, while our study focused on PD-L1 and TILs, emerging evidence suggests that combination strategies targeting multiple immune checkpoints and cytokines may overcome resistance mechanisms [47]. The development of predictive models incorporating immune parameters, similar to those used for treatment response [48], could help optimize patient selection for immunotherapy. Larger prospective studies with multiparameter immune profiling are needed to validate our findings and establish standardized approaches for evaluating the immune landscape in primary versus metastatic breast cancer, particularly in patients receiving various treatment modalities and with different metastatic sites.
Conclusions
To conclude, our study revealed relationships between TILs, PD-L1 expression, and clinico-immunological characteristics in primary breast cancer. Both tumor cell PD-L1 and PD-L1+ TIL expression levels were higher in untreated primary tumors compared to recurrent/metastatic lesions. Similarly, TIL density was lower in recurrent/metastatic sites, suggesting immune escape as a key mechanism in post-surgical recurrence. High PD-L1+ TIL expression correlated positively with tumor grade, tumor cell PD-L1 expression, and tertiary lymphoid structure development. Tumor cell PD-L1 was positivity associated with worse disease-free and overall survival, indicating greater malignant potential and poorer prognosis, while PD-L1+ TIL expression showed no prognostic significance. These findings suggest that patients with tumor cell PD-L1 expression may particularly benefit from PD-1/PD-L1 pathway inhibition through enhanced immune activation.


