Overview of tumor-infiltrating B lymphocytes
Classification of B cell subpopulations and the emerging range of tumor-infiltrating B lymphocyte phenotypes
Early studies of human cancers demonstrated that the tumor-infiltrating B lymphocytes (TIL-B) phenotype includes naive B cells, activated B cells, memory B cells, germinal center B cells, plasma cells (PC) and their intermediates. In general, the described TIL-B phenotype aligns with canonical B cell subsets identified in healthy donors, yet shows greater heterogeneity in certain subpopulations [1]. Memory B cells and plasma cells exhibit the most pronounced heterogeneity [2, 3], with plasma cell heterogeneity notably reflected in their antibody class switching, closely linked to tumor immunity. TIL-B can be broadly classified into antibody-secreting tumor infiltrating plasma cells (TIL-PLC), regulatory B cells (Breg) and antigen-presenting B cells (APB) based on their function within the tumor [4], each potentially exerting differing impacts on the tumor. The highly specific recognition capability of tumor-associated antigen (TAA) positions TIL-PLC as central players in the anti-tumor immune response. Studies have confirmed that plasma cells are capable of producing antibodies that specifically recognize and bind to TAA across a variety of cancers [5]. Breg, in particular, have garnered significant attention, sharing similarities with those observed in autoimmunity, and can be derived from memory cells, plasmablasts, and PC [4]. The differentiation of Breg is considered a crucial source of B cell-mediated tumor-promoting effects [4], often characterized by their competence in producing interleukin (IL)-10 and transforming growth factor β (TGF-β). Studies have demonstrated that in vitro stimulation with recombinant CD40 ligand and toll-like receptor agonists can induce the production of IL-10 by Breg [6]. B10 cells are a significant subset within Breg, known for their tumor-promoting capabilities through IL-10 production and involvement in various immune disorders. The latest studies suggest that B-1 cells have also become potential players in tumor immunity in various subpopulations of TIL-B [7]. Furthermore, multiple factors influence the TIL-B phenotype, including the B cell’s microenvironment, disease stage, and tumor type. A study highlighted a transition from naive to PC-like B cells in advanced lung cancers [8], indicating the importance of considering B-cell subpopulation changes due to disease progression or type in future research.
Tumor-infiltrating B lymphocyte community
Within the complex ecosystem of the tumor microenvironment (TME), TIL-B establish their presence in distinct niches, each characterized by its unique organization and cellular composition. These areas not only indicate the adaptive localization of TIL-B in response to the evolving tumor landscape but also highlight their multifaceted roles in cancer immunity. Here we describe the three primary regions where TIL-B exert their influence.
Tertiary lymphoid structures
Tertiary lymphoid structures (TLS) are lymph node-like structures and ectopic lymphoid organs. TIL-B are often colocalized with T cells or other immune cells (such as dendritic cells) in the organized lymphoid aggregate [9]. They can be actively involved in initiating and maintaining adaptive immune responses. Some researchers believe that any lymphoid aggregates can be described as TLS, but strictly speaking, although TLS share many similarities with lymph nodes, their structural differences may still have a significant impact on the TIL-B response. For example, TLS may produce a TIL-B response with different antigenic specificity, whereas lymph nodes cannot. In conclusion, TLS may induce auto-reactive B cells and PC, which underlie autoimmune-mediated toxicity associated with immune checkpoint blockade. But on the other hand, TLS may relax the self-tolerance mechanism and allow the body to produce auto-reactive antibodies as a way to counteract neoantigens and other tumor-specific antigens [10]. The presence of TLS is often correlated with improved patient prognosis, underscoring their significance in fostering a potent antitumor immune response.
Lymphocyte-myeloid aggregates
Representing a less organized but equally pivotal form of immune cell congregation, lymphocyte-myeloid aggregates (LMAs) comprise a heterogeneous mix of TIL-B, T cells, and macrophages [11]. These collections facilitate dynamic interactions between immune cells and the tumor cells, allowing for the exchange of cytokines, chemokines, and other mediators that shape the immune landscape. The formation of LMAs reflects the immune system’s adaptability in ascending a response despite the suppressive conditions influenced by the TME [12, 13].
Intra-epithelial infiltrates
In the fight against tumor cells, TIL-B, along with T cells and macrophages, infiltrate the epithelial layers of the tumor, positioning themselves within striking distance of their target. This infiltration indicates an aggressive stance by the immune system, aiming to suppress tumor growth and prevent metastasis. The interaction of TIL-B with tumor cells in this region is crucial for the direct killing of tumor cells, antibody-dependent cellular cytotoxicity (ADCC), and the regulation of a localized immune response.
In each of these regions, TIL-B engage in a complex interplay with other immune cells and the tumor itself, shaping the course of the immune response through direct contact and mediated signaling. The strategic positioning of TIL-B within these distinct areas of the TME highlights their multi-function and critical role in modulating cancer progression and the effectiveness of immunotherapeutic strategies. Understanding the dynamics of the TIL-B community within these environments opens new ways for enhancing their potential in cancer treatment and provides insights into the mechanisms of successful antitumor immunity [10] (Table 1).
Table 1
Three main regions in which tumor-infiltrating B lymphocytes (TIL-B) exert their effects
| Variable | TLS | LMA | Intra-epithelial infiltrates |
|---|---|---|---|
| Structural organization | Organized lymphoid aggregate [9] | Heterogeneous mixture [11] | Infiltrate into the epithelial layers of the tumor |
| Composition of immune cells | TIL-B, T cells, dendritic cells [9] | TIL-B, T cells, macrophages [11] | TIL-B, T cell, macrophages |
| Function | Initiation and maintenance of adaptive immune responses | Facilitate dynamic interactions between immune cells and the tumor cells | Suppress tumor growth and prevent metastasis [10] |
| Immune response | Fostering a robust anti-tumor immune response [10] | Facilitate dynamic interactions between immune cells and the tumor cells [12] | Direct killing of tumor cells, ADCC, and the regulation of a localized immune response |
| Effects on tumors | May be associated with autoimmune-mediated toxicity associated with immune checkpoint blockade [10] | Subject to TME suppression conditions [12] | Directly acting on tumor cells [10] |
Fig. 1
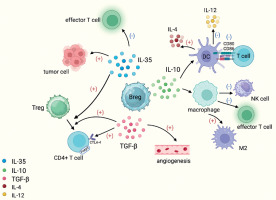
Breg promote tumor development by secreting immunosuppressive cytokines. Breg secrete IL-10, make DC overexpress IL-4 and reduce production of IL-12, downregulate CD80/CD86 expression on DC surface and thereby reduce T cell activation, and also promote differentiation of tumor-associated macrophages into the M2 phenotype and ultimately suppress effector T cells and NK cells. IL-35 secreted by Breg can promote the differentiation of T cells into Treg cells, induce effector T-cell depletion, and inhibit apoptosis of tumor cells. TGF-β secreted by Breg can transform CD4+ T cells into Treg and is also able to induce angiogenesis
Immunological mechanism of tumor-infiltrating B lymphocytes
The immunological mechanisms of TIL-B can be broadly classified into anti-tumor and pro-tumor immunity. The effect of TIL-B on tumor growth or inhibition depends mainly on the tumor microenvironment as well as on certain B-cell subsets.
Tumor suppressive effect of tumor-infiltrating B lymphocytes
Generation of complement signals or co-stimulatory signals
It has been shown that inducible costimulatory molecular ligand (ICOSL) in B cells enhances antitumor immunity by enhancing the effector to regulatory T cell ratio and strengthening the effect of T cells. The distinct ICOSL signature on B cells is a result of complement-CR2 signaling, initiated in response to immunogenic cell death. Furthermore, the ICOS-ICOSL co-stimulatory pathway is instrumental in triggering the secretion of T helper cell 1 (Th1) and T helper cell 2 (Th2) cytokines, thereby amplifying the immune response through the potentiation of CD28/B7-1/B7-2 signaling pathways. This mechanism is crucial for the activation of T cell-dependent B cell responses. Notably, an increased presence of ICOSL-expressing B cells within the tumor microenvironment has been associated with significant improvements in both disease-free and overall survival among breast cancer patients undergoing neoadjuvant chemotherapy, underscoring the therapeutic potential of targeting this pathway.
The synergistic effect of B cells and T cells
Many studies have shown that B cells and T cells play a joint role in anti-tumor immunity. In addition to supporting the function of T cells in immune killing through the induction of co-stimulator (ICOS) ligands CD80 and CD86 [9], B cells can also play a key role in the tumor immune response by triggering the tumor killing action of T cell through co-stimulatory signals (such as CD40/CD40L) [14]. B cells were also found to promote TLS formation through the secretion of chemokine (C-X-C motif) ligand 13 (CXCL13) and cytotoxic factors [14]. All of these results suggest that B cells can achieve tumor suppression by activating and inducing T cells.
Release of cytokines
Tumor-infiltrating B lymphocytes are responsive to B cell receptor (BCR) stimulation ex vivo, express activation markers, and produce cytokines and immunoglobulins (Igs) despite reduced expression of the antigen-presenting molecules human leukocyte antigen-DR (HLA-DR) and CD40 [15]. In addition, the cytokines produced not only promote B cell development and isotype switching but also play a major role in supporting the differentiation and proliferation of T cells and natural killer cells [16].
Secretion of specific antibodies and antibody-dependent intracellular neutralization
According to the research of Fridman, TIL-B can induce apoptosis of tumor cells by self-producing antibodies to proteins on the surface of tumor cells. Meanwhile, TLS in tumors can promote antibody production and maintain B-cell maturation, which can have a direct anti-tumor effect [17]. In addition, antibody-dependent intracellular neutralization may also help to exert tumor suppressive effects. Antibody-dependent intracellular neutralization is a process that utilizes antibodies to induce intracellular proteasomal degradation [18, 19]. By studying TIL-B-derived IgG in lung cancer, researchers demonstrated that the degradation of tumor cells can be promoted through antibody-dependent intracellular neutralization [8].
Involvement in antigen presentation
B cells within the tumor TLS act as specialized antigen-presenting cells (APC) that can achieve antigen presentation through direct or indirect cross-presentation. They exhibit high expression levels of major histocompatibility complex (MHC) class I and II molecules, along with co-stimulatory molecules, thereby processing and presenting antigens to both CD8+ and CD4+ T cells [20]. This process not only shapes but also amplifies the T cell responses, playing a crucial role in the immune system’s fight against cancer. Recently, it has also been reported that tumor-specific B-cell presentation of antigens can be observed in mice that have been used with model antigens, leading to a T-cell response to the tumor [21]. In addition, other studies further reinforce this perspective. For example, B cells that are activated by CD40 and then pulsed with exogenous antigens develop functional and molecular abilities that can present MHC class II epitopes, thus making them antigen-presenting cells that produce specific T cells [22]. Wennhold also showed that CD40-activated B cell are effective antigen-presenting cells that can activate and expand naive and memory CD4+ and CD8+ T cells and homing to secondary lymphoid organs [23].
Cytotoxic effects
Fas/FasL and perforin pathways can enhance anti-tumor immune responses. It has been found that tumor-draining lymph node (TDLN) B cells can express FasL and act directly on 4T1 tumor cells after in vitro bacterial lipopolysaccharide/anti-CD40 activation, and the interaction between TDLN B cells and 4T1 leads to increased levels of FasL expression on the surface of B cells, while tumor cells express high levels of Fas. Therefore, the effector B cells directly kill the target cells through the Fas/FasL pathway [24]. In addition, some researchers found that if both FasL and chemokine (C-X-C motif) receptor 4 (CXCR4) action pathways were blocked, the killing effect of B cells on tumor cells was inhibited, which also suggested that both Fas/FasL and CXCL12/CXCR4 action pathways were involved in the killing effect of TDLN B cells on 4T1 cells. In contrast, the transwell assay showed that TDLN B cells can produce perforin, which allows them to directly kill tumor cells without contacting them and subsequently enhance their cytotoxic effect on tumor cells [25]. Although some scholars believe that there is no direct evidence so far that TIL-B-derived antibodies induce ADCC, we can still take this direction as an entry point for tumor immunology therapy.
Transcytosis
Some researchers have suggested that immunoglobu- lin A (IgA) transmigration could serve as a novel mechanism for TIL-B-mediated anti-tumor immunotherapy. The surface of mucosal epithelial cells was shown to express polymeric immunoglobulin receptors (pIgR) that bind to and are internalized by PC-derived IgA. In ovarian tumor lines, in vitro exposure of IgA by pIgR inhibited oncogenic signaling and promoted T cell-mediated killing through a pIgR-dependent mechanism [26].
Tumor-suppressive effects of B-1 cells
In the realm of innate immune responses, B-1 cells play a pivotal role in the antitumor immune response upon encountering stimuli triggered by toll-like receptor (TLR) agonists or microbial pathogens, which enables them to produce a substantial amount of natural antibodies (NAbs), predominantly IgM, that are crucial for defending against infections [27]. These characteristics position B-1 cells as a vital component of the first line of immune defense. B-1 cells exhibit their antitumor activity by secreting NAbs that specifically target mouse and TAA [7]. These NAbs can eliminate tumor cells through various mechanisms, including ADCC, complement-dependent cytotoxicity (CDC), and complement-independent cytotoxicity (CIC). Furthermore, natural IgM plays a key role in recognizing tumor neoantigens and activating adaptive immune responses against tumor cells [7]. Studies also suggest that B-1 cells can function as APC and phagocytes, yet their potential in this regard warrants further investigation and exploration.
Tumor-promoting effect of tumor-infiltrating B lymphocytes
Immunosuppressive cytokines
Breg, a subpopulation of TIL-B, is a subpopulation of B cells similar to regulatory T cells (Treg). It is an immunosuppressive cell that negatively regulates the immune response by releasing immunosuppressive factors such as IL-10, IL-35, TGF-β and even gamma-aminobutyric acid (GABA). Interleukin 10 can lead to overexpression of IL-4 by dendritic cells (DC) and a reduction in the production of IL-12, downregulating the expression of CD80/CD86 on the surface of DC, thereby reducing the activation of T cells. Additionally, IL-10 inhibits the activity of inflammatory cells such as macrophages and can promote the differentiation of tumor-associated macrophages into the M2 phenotype, ultimately suppressing the activity of effector T cells and natural killer cells [28, 29]. Breg have been detected in the peripheral blood of gastric cancer patients and mediate tumor cell escape through IL-10 signaling [30]. Interleukin 35 plays a significant role in immune suppression by promoting the differentiation of T cells into Treg, inducing exhaustion in effector T cells, inhibiting apoptosis in tumor cells, and downregulating the antigen-presenting capacity of B cells [31]. In a clinical study of chronic myeloid leukemia, tumor-produced TGF-β induced an immunosuppressive phenotype in Breg, resulting in accelerated tumor progression [9]. TGF-β plays a multifaceted role in promoting the proliferation and migration of endothelial cells, thereby inducing angiogenesis [32]. Additionally, TGF-β is capable of converting CD4+ T cells into Treg, limiting the proliferation and function of effector T cells, and enhancing the expression of FoxP3 and CTLA-4, which in turn promotes tumor growth and metastasis [33].
Interaction with T cells
The interaction between T cells and B cells sometimes has an anti-tumor effect, but sometimes promotes a tumor-promoting effect. It has been shown that the suppressive effect of Breg is directly mediated by the secretion of IL-10 and the interaction of B cells with pathogenic T cells; thus Breg play a tumor-promoting role while promoting the development of Treg [24]. It has also been shown that Breg can play an immunosuppressive role by inhibiting the secretion of interferon γ (IFN-γ) and tumor necrosis factor α (TNF-α) by CD4 Th cells and also inhibiting the secretion of cytokines by T cells to promote the conversion of T cells into Treg [34].
Trigger programmed death cell
This tumor-promoting subpopulation of B cells, which constitutively express high levels of programmed death-1 (PD-1), acquires regulatory functions when encountering programmed cell death ligand-1 (PD-L1)(+) cells or experiencing PD-1 triggering, and thus suppresses tumor-specific T cell immunity and promotes tumor cell growth through IL-10 signaling [29]. This is based on the blocking of the activation of immunosuppressive receptors.
B10 cells
Recent investigations have increasingly highlighted the pivotal role of B10 cells, a subset of B cells, as crucial suppressors within the complex network of the body’s antitumor immune defense. These cells collaborate extensively with a diverse array of immune cells spanning both the innate and adaptive immune responses, orchestrating a conducive environment that inadvertently promotes tumor growth and facilitates metastasis. By engaging in comprehensive interactions, B10 cells not only amplify the regulatory mechanisms that suppress anti-tumor immunity but also enhance conditions that favor tumor progression and the spread of metastatic cells. This multifaceted involvement of B10 cells in modulating the immune response underscores their significant role in cancer biology, offering new insights into the dualistic nature of immune responses in the context of cancer and highlighting potential targets for therapeutic intervention. Their ability to interface with various immune cell types underscores the complexity of tumor-immune dynamics, suggesting that modulating B10 cell activity could provide a novel way for enhancing the efficacy of cancer immunotherapies [35].
Antibodies produced by plasma cells
Antibodies produced by PC may have pro-tumor effects, mainly including IgG variants and IgA. In a colorectal cancer study, IgG4 induced activation of M2 macrophages, impairing anticancer effects and promoting tumor development [36]. Regarding the tumor-promoting effect of IgA, it has been found that the concentration of IgA is positively correlated with a poor prognosis in hepatocellular carcinoma [37, 38]. This also suggests that IgA can modulate the immune function of T cells and lead to decreased anti-tumor capacity. This evidence highlights the potential for targeted therapeutic strategies that alleviate the pro-tumor effects of specific antibody classes.
Tumor-promoting effects of B1 cells
B1 cells not only exert tumor-suppressing effects, but also promote tumor growth at times. Recent studies have indicated that B-1 cells display immunosuppressive functions that may undermine antitumor immunity or facilitate tumor progression [7]. The interaction between B-1 cells and tumor cells enhances their ability to secrete IL-10, which in turn boosts the vitality, proliferation, and resistance to cell death of B-1 cells [7]. In addition to IL-10, a variety of immunosuppressive cytokines including TGF-β and IL-35 are associated with the suppression of antitumor immunity [39, 40].
Tumor-infiltrating B lymphocytes-based therapeutic approach
Most of the TIL-B-based tumor immunotherapies are still in the preclinical research stage. Currently, there are two main directions: one is to enhance the anti-tumor effect of TIL-B, and the other is to inhibit the pro-tumor effect of Breg and others in vivo.
Enhancement of the antitumor effect of tumor-infiltrating B lymphocytes
Tumor-infiltrating B lymphocytes works with chemotherapy and targeted drugs
Studies by Lu et al. and Hollern et al. showed that the density of TIL-B was positively correlated with chemotherapy response in a mouse model [34, 41], and TIL-B could improve the efficacy of chemotherapeutic drugs. Meanwhile, chemotherapy can further increase TIL-B density, co-stimulatory molecule expression, and induce TLS [34, 42, 43]. In mouse melanoma models, STING agonists can contribute to the formation of immature TLS and may be used in combination with drugs that induce TLS maturation [44]. Another study showed that the efficiency of chemotherapy was significantly higher in the lymphocyte group with higher infiltration than in the low infiltration group, which suggests that the infiltration degree of TIL-B can also be used as an important reference indicator for the effectiveness of chemotherapy [45, 46].
The effect of antibodies
Several clinical studies have demonstrated a significant correlation between antibodies produced by TIL-B in the treatment and survival of tumor patients. For example, overall survival of breast cancer patients after chemotherapy showed a significant correlation with mucin-specific IgG antibody levels [47], while IgG+ and IgM+ B lymphocytes were denser in cancers with a high degree of TIL-B infiltration compared to normal mammary glands [48]. In triple-negative breast cancer patients, Ig gene expression was positively correlated with disease-free survival [49], which further indicates the important role of antibodies in tumor treatment.
Relay transfer B-cell therapy
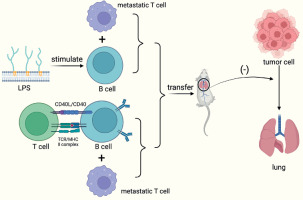
Targeted approaches that augment conventional B cells with B cell ligand stimulation are effective in suppressing tumor growth, while CpG-activated B cells and tumor-draining lymph node B cells (TDLN-B cell) also show enhanced immune properties after metastasis. In a mouse model of breast cancer constructed by researchers, in vitro bacterial lipopolysaccharides (LPS) and CD40-stimulated B cells could limit lung metastasis upon metastasis, and the effect was more pronounced when combined with metastatic T cells [50].
Activation of anti-tumor B cells
Direct activation of B cells in vivo can also show potent tumor suppression. For example, CXCL13-coupled CpG oligonucleotide (CpG-ODN) stimulation of CXCR5-expressing B cells can activate antitumor B cells. Meanwhile, non-specific immune activators such as cytokines and endotoxins can also be used to activate B cells. However, this method takes a long time for treatment, has more side effects, and its application is more limited [51].
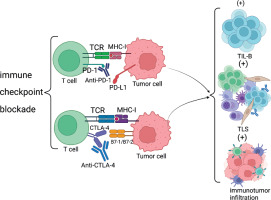
Immune checkpoint blockade
It has been shown that targeting immune checkpoint blockade with PD-1 or PD-L1 may directly affect TIL-B. There is substantial evidence that anti-CTLA-4 and anti- PD-1 antibody blockade has a positive effect on TIL-B and TLS. In a mouse melanoma model constructed by researchers, blockade of immune checkpoints was found to improve the number and characterization of TLS, along with a greater improvement in the efficacy of melanoma [52]. In other tumor models, an increase in the number of TLS and improved tumor control after treatment with anti-PD-1 antibody were also observed. This also demonstrates that immune checkpoint blockade can offer a new approach for the treatment of tumors.
Inhibition of the pro-tumor effects of Breg
Studies have shown that Breg may promote tumor growth, so researchers have attempted to treat tumors by depleting Breg. Current research has found that anti-CD20 antibodies can help deplete Breg and improve the survival rate of tumor patients [53]. But at the same time, the method is time-sensitive. In an established mouse model, it was found that treatment with anti-CD20 antibody after tumor establishment resulted in increased metastasis of tumor cells in mice, while using it just before tumor attack resulted in decreased metastasis. This may be due to the depletion of immunostimulatory CD20+ B cells and anti-CD20 antibody-mediated enrichment of CD20 Breg after tumor establishment. The same results were not obtained after applying the anti-CD20 antibody depletion Breg cell strategy, possibly because this strategy was ineffective for Breg subpopulation treatment [54]. Therefore, selective depletion of Breg may be a more effective approach compared to the depletion of pan-B cells using anti-CD20 antibodies.
Another way that Breg can be depleted is by targeting CpG-ODN with CXCR5, inactivating resveratrol with stat3, or depleting IL-10, which can inhibit Breg-dependent Treg transformation [55]. This will lead to the depletion of Breg and exert a pro-tumor effect.
The role of tumor-infiltrating B lymphocytes as a biomarker in auxiliary diagnosis
Tumor-infiltrating B lymphocytes serve as multidimensional biomarkers in cancer patients, particularly in auxiliary diagnosis and prognosis within the context of tumor treatment. TIL-B facilitate T cell-mediated antitumor immunity through their unique antigen presentation mechanisms [10], which aid physicians in understanding the immune status of tumors, thereby enabling the formulation of more effective treatment strategies. Of course, their significance in prognosis should not be overlooked, often providing physicians with vital information regarding the disease progression and potential treatment responses of patients [45].
Clinical and prognostic significance of tumor-infiltrating B lymphocytes
Researchers have studied a variety of tumors including breast cancer, lung cancer, hepatocellular carcinoma, cutaneous melanoma, and epithelial ovarian cancer, thus providing insight into the therapeutic and prognostic significance of TIL-B.
Clinical significance
By analyzing a large number of samples, researchers found that TIL-B density was significantly increased in tumor tissues compared to normal tissues [15]. And high density of TIL-B tended to be significantly associated with higher survival, disease-free survival (DFS), overall survival (OS), and polymerase chain reaction (PCR) rates. And in tumor tissues, the infiltration of TIL-B is significantly higher than in surrounding tissues or other non-tumor tissues, which may be because the localization of TIL-B would be highly controlled by signals or stimuli in the tumor microenvironment [50]. In recent years, more and more studies have also shown that TIL-B can regulate T cell activation, expansion, and memory formation, both of which can act synergistically on tumor tissues to prolong patient survival [50]. For example, in their observation of cutaneous melanoma, researchers found that patients with high infiltrating B cells and high activated T cells exhibited the highest 5-year survival in the cohort, while patients from the low B cell/low activated T cell subgroup exhibited the worst survival among all subgroups [56]. Also, well-organized TIL is associated with sustained humoral immune responses and elevation, suggesting a clinically important role for TIL-B in antitumor immunity.
Prognostic significance
Tertiary lymphoid structures represent a critical subset of TIL-B, emerging as a significant prognostic marker within the tumor microenvironment. The antibodies produced by PC can also be used as a prognostic indicator [9]. The presence, abundance, and maturity of TLS not only serve as a barometer for the immune system’s engagement with the tumor but also have been identified as potent predictors of patient outcomes. Remarkably, the antibodies generated by PC, particularly those associated with TLS, have been acknowledged as reliable prognostic indicators, offering a new insight into the tumor’s future development [57]. The complicated relationship between the prognostic implications of TIL-B and its spatial distribution within tumor tissues has been well documented. Research on breast cancer progression showed that a dispersed distribution of TIL-B correlates with a notably favorable prognosis, in contrast to a more confined distribution, which suggest a poorer outcome. This observation underscores the ability of a widely distributed B cell population to assess tumor dissemination more effectively, thereby leading to a more optimistic prognosis.
Moreover, the infiltration of TIL-B within the tumor and the prognostic outcome are also related. Generally, high infiltration of TIL-B is associated with a better prognostic profile [45]. For example, in hepatocellular carcinoma, researchers evaluated the relationship between the expression of six B cell-related genes and the survival and prognosis of hepatocellular carcinoma patients with the help of the TCGA (The Cancer Genome Atlas) database. High infiltration and high expression of these six B cell-related genes were found to be associated with improved survival and prognosis of hepatocellular carcinoma [58].
Although TIL-B is associated with a better prognostic profile in most tumor tissues, the prognostic significance of TIL-B varies between tumor types or different periods of the same tumor. For example, no significant correlation between TIL-B and prognosis was observed in small cell carcinoma of the lung, while most studies on non-small cell carcinoma revealed a better prognostic outcome [50]. This also indicates that TIL-B has different prognostic significance in different situations, and further studies are needed.
Significance for the treatment of cancer patients
Recent advancements in the study of TIL-B have presented multifaceted potential benefits for the treatment of cancer patients. Firstly, TIL-B promote antitumor immunity by modulating T cell activation, expansion, and memory formation, and the synergistic action of these two cell types helps to prolong patient survival [59]. Concurrently, the interaction between TIL-B and T cells in TLS may stimulate functional and protective cooperation, which is conducive to the activation of adaptive immune responses and the generation of immune memory [60], potentially having a significant impact on responses to immunotherapy. Moreover, the presence of PD-1+ and PD-L1+ TIL-B in various human cancers has been reported, preliminarily indicating that TIL-B may be directly affected by PD-1 and/or PD-L1-targeted immune checkpoint blockade [10]. This suggests that chemotherapy or targeted therapy aimed at specific B cell subsets may hold value, offering possibilities for the development of new treatment strategies. The role of TIL-B as novel biomarkers should not be overlooked, as they not only assist in predicting treatment outcomes and patient prognosis [47], but also guide the formulation of treatment strategies and the development of new therapeutic targets. The diversity and functional status of TIL-B, including effector B cells and Breg subsets, also provide new perspectives and potential therapeutic targets for cancer treatment.
Conclusions
Tumor-infiltrating B lymphocytes constitute a pivotal yet underexplored element within the tumor microenvironment, exerting a significant influence on cancer immunotherapy. The functional diversity of TIL-B, which includes their dual roles in both promoting and suppressing tumor growth, as well as their potential as biomarkers and therapeutic targets, underscores the necessity for further research in this domain. TIL-B secrete and release cytokines and antigens specific to tumors, interact with immune cells such as T cells, and exhibit distinct functions in various ecological niches. Studies focusing on these aspects may uncover novel mechanisms of immune regulation, thereby laying the theoretical groundwork for the development of combined immunotherapy strategies.
Although the mechanisms of TIL-B action in tumor immunity remain incompletely elucidated, their interactions with tumor antigens and differentiation processes within the tumor microenvironment may account for their functional heterogeneity. Addressing this fundamental question could significantly advance our understanding of the role of B cells in tumor immune responses.
It is noteworthy that the distribution, function, and impact of TIL-B on tumor prognosis and therapeutic response may vary significantly across different types of cancer. This necessitates consideration of tumor type heterogeneity in research, with particular attention to their potential application in high-mortality cancers such as lung and pancreatic cancers. Concurrently, advancements in gene-editing technologies, such as CRISPR/Cas9, enable researchers to genetically modify TIL-B to enhance their ability to recognize and attack tumor cells. Furthermore, developments in genomic sequencing technologies have allowed researchers to more accurately identify tumor-specific antigens, thereby improving the precision and efficacy of TIL-B therapies and providing personalized and precise treatment plans for patients. As research continues to advance, we can anticipate the development of more precise cancer immunotherapy strategies. This will not only bring groundbreaking progress to the field of oncology but also offer renewed hope to patients worldwide.
In summary, a thorough comprehension of the functional heterogeneity of TIL-B and their molecular regulatory mechanisms is invaluable for propelling the advancement of the field of cancer immunotherapy. Future research should further investigate the mechanistic roles of TIL-B within the tumor microenvironment. Exploring the combination of TIL-B with immune checkpoint inhibitors, cytokine therapies, or other immunotherapies will be instrumental in elucidating the synergistic effects of TIL-B in tumor control, overcoming the immune evasion mechanisms of tumors, and providing a theoretical foundation for the development of new therapeutic strategies. Concurrently, attention must be paid to the development and validation of new biomarkers to predict patient responses to TIL-B-related treatments. These biomarkers can assist physicians in selecting patients who are most likely to benefit from TIL-B therapies and provide a basis for personalized treatment. The potential of TIL-B as therapeutic targets should not be overlooked, including their roles in tumor immune editing and tumor heterogeneity, thereby facilitating the development of novel immunotherapies targeting specific B cell subsets. Currently, deciphering and more precisely characterizing the various cellular neighborhoods of TIL-B through multiple immunohistochemical methods is of significant importance for a deeper understanding of the tumor immune microenvironment, the development of new therapies, and the enhancement of treatment efficacy. Additionally, fostering seamless integration between basic research, clinical development, and patient care is essential. This integration is crucial for achieving the full potential of TIL-B in oncology and optimizing cancer immunotherapy strategies. Through interdisciplinary collaboration and innovative research approaches, we can expect more breakthroughs in the field of tumor immunotherapy, ultimately achieving more effective cancer treatment and significant improvements in patient survival.